Hot And Cold Packs Chemical Reaction . How can something produce heat or cold without any microwaving or refrigeration involved? The sodium thiosulfate needs energy to. commercial heat packs (containing iron and water, or supersaturated sodium acetate) and cold packs (various ammonium salts) can. have you ever used a hot pack to warm your hands or a cold pack on an injury? in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction that. learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. in an endothermic reaction, a substance takes heat from its surroundings. hot pack cold pack (conservation of energy in chemical reactions, heat of solution, hess's law of heat summation) |.
from www.chegg.com
The sodium thiosulfate needs energy to. commercial heat packs (containing iron and water, or supersaturated sodium acetate) and cold packs (various ammonium salts) can. How can something produce heat or cold without any microwaving or refrigeration involved? in an endothermic reaction, a substance takes heat from its surroundings. hot pack cold pack (conservation of energy in chemical reactions, heat of solution, hess's law of heat summation) |. have you ever used a hot pack to warm your hands or a cold pack on an injury? learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction that.
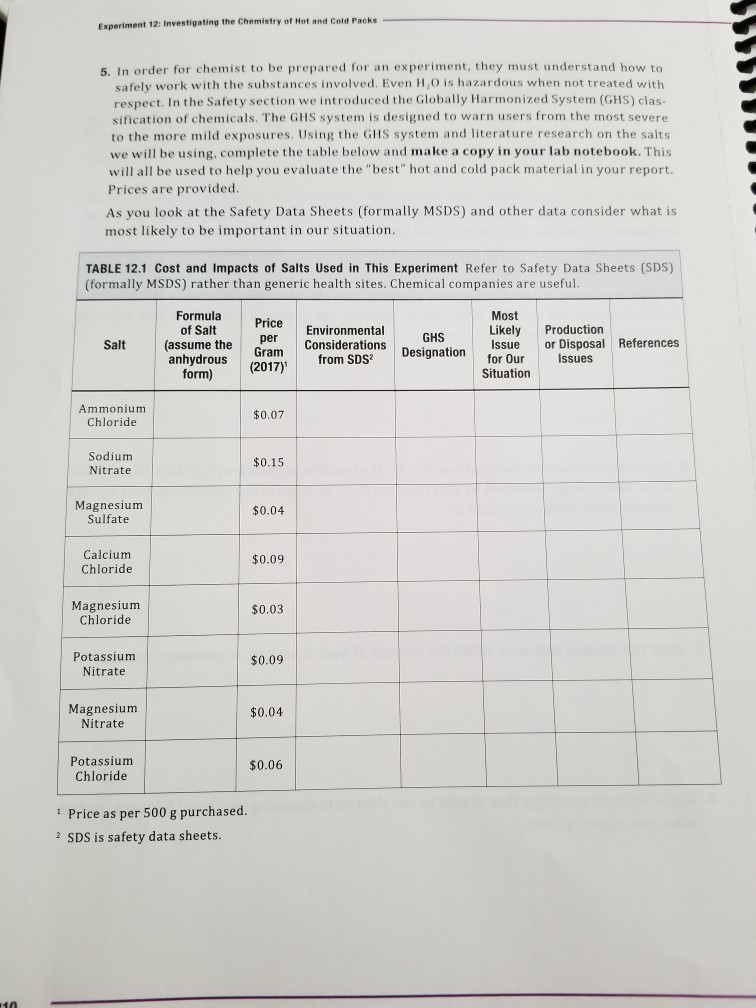
Solved e Chemistry of Hot and Cold Packs Experiment 12
Hot And Cold Packs Chemical Reaction commercial heat packs (containing iron and water, or supersaturated sodium acetate) and cold packs (various ammonium salts) can. How can something produce heat or cold without any microwaving or refrigeration involved? have you ever used a hot pack to warm your hands or a cold pack on an injury? hot pack cold pack (conservation of energy in chemical reactions, heat of solution, hess's law of heat summation) |. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction that. in an endothermic reaction, a substance takes heat from its surroundings. commercial heat packs (containing iron and water, or supersaturated sodium acetate) and cold packs (various ammonium salts) can. The sodium thiosulfate needs energy to. learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions.
From www.scribd.com
Making Hot and Cold Packs 2 PDF Ion Chemical Reactions Hot And Cold Packs Chemical Reaction How can something produce heat or cold without any microwaving or refrigeration involved? commercial heat packs (containing iron and water, or supersaturated sodium acetate) and cold packs (various ammonium salts) can. in an endothermic reaction, a substance takes heat from its surroundings. have you ever used a hot pack to warm your hands or a cold pack. Hot And Cold Packs Chemical Reaction.
From spark.adobe.com
Demonstrating The Science Of A Cold Pack Hot And Cold Packs Chemical Reaction have you ever used a hot pack to warm your hands or a cold pack on an injury? in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction that. in an endothermic reaction, a substance takes heat from its surroundings. How can something produce heat or. Hot And Cold Packs Chemical Reaction.
From sciencenotes.org
Hand Warmer Chemistry Easy Chemical Hot Packs Hot And Cold Packs Chemical Reaction The sodium thiosulfate needs energy to. hot pack cold pack (conservation of energy in chemical reactions, heat of solution, hess's law of heat summation) |. How can something produce heat or cold without any microwaving or refrigeration involved? learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. . Hot And Cold Packs Chemical Reaction.
From fphoto.photoshelter.com
science chemical reaction endothermic cold pack Fundamental Hot And Cold Packs Chemical Reaction commercial heat packs (containing iron and water, or supersaturated sodium acetate) and cold packs (various ammonium salts) can. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction that. learn about the chemical reactions used to make cold packs and which chemicals to mix to cause. Hot And Cold Packs Chemical Reaction.
From www.humed.lk
Hot Cold Pack HuMed Hot And Cold Packs Chemical Reaction have you ever used a hot pack to warm your hands or a cold pack on an injury? The sodium thiosulfate needs energy to. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction that. How can something produce heat or cold without any microwaving or refrigeration. Hot And Cold Packs Chemical Reaction.
From studylib.net
The Chemistry of Hot and Cold Packs Hot And Cold Packs Chemical Reaction commercial heat packs (containing iron and water, or supersaturated sodium acetate) and cold packs (various ammonium salts) can. The sodium thiosulfate needs energy to. hot pack cold pack (conservation of energy in chemical reactions, heat of solution, hess's law of heat summation) |. How can something produce heat or cold without any microwaving or refrigeration involved? learn. Hot And Cold Packs Chemical Reaction.
From brooklynewtortega.blogspot.com
Cold Pack Endothermic or Exothermic BrooklynewtOrtega Hot And Cold Packs Chemical Reaction learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. in an endothermic reaction, a substance takes heat from its surroundings. How can something produce heat or cold without any microwaving or refrigeration involved? have you ever used a hot pack to warm your hands or a cold. Hot And Cold Packs Chemical Reaction.
From fixlibrarynadred27.z14.web.core.windows.net
Exothermic And Endothermic Diagrams Hot And Cold Packs Chemical Reaction have you ever used a hot pack to warm your hands or a cold pack on an injury? in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction that. in an endothermic reaction, a substance takes heat from its surroundings. hot pack cold pack (conservation. Hot And Cold Packs Chemical Reaction.
From pressbooks.bccampus.ca
3.9 Energy in Chemical Reactions Human Biology Excerpts for BBIO 053 Hot And Cold Packs Chemical Reaction How can something produce heat or cold without any microwaving or refrigeration involved? have you ever used a hot pack to warm your hands or a cold pack on an injury? hot pack cold pack (conservation of energy in chemical reactions, heat of solution, hess's law of heat summation) |. learn about the chemical reactions used to. Hot And Cold Packs Chemical Reaction.
From www.sliderbase.com
Oxidation/Reduction Reactions Hot And Cold Packs Chemical Reaction The sodium thiosulfate needs energy to. commercial heat packs (containing iron and water, or supersaturated sodium acetate) and cold packs (various ammonium salts) can. learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. How can something produce heat or cold without any microwaving or refrigeration involved? hot. Hot And Cold Packs Chemical Reaction.
From www.slideserve.com
PPT Hot Packs and Cold Packs PowerPoint Presentation, free download Hot And Cold Packs Chemical Reaction commercial heat packs (containing iron and water, or supersaturated sodium acetate) and cold packs (various ammonium salts) can. learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. How can something produce heat or cold without any microwaving or refrigeration involved? in this lesson plan, students will explore. Hot And Cold Packs Chemical Reaction.
From www.slideserve.com
PPT CHEMICAL REACTIONS PowerPoint Presentation, free download ID Hot And Cold Packs Chemical Reaction in an endothermic reaction, a substance takes heat from its surroundings. hot pack cold pack (conservation of energy in chemical reactions, heat of solution, hess's law of heat summation) |. learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. have you ever used a hot pack. Hot And Cold Packs Chemical Reaction.
From letstalkscience.ca
The Cold Pack A Chilly Example of an Endothermic Reaction Let's Talk Hot And Cold Packs Chemical Reaction hot pack cold pack (conservation of energy in chemical reactions, heat of solution, hess's law of heat summation) |. How can something produce heat or cold without any microwaving or refrigeration involved? commercial heat packs (containing iron and water, or supersaturated sodium acetate) and cold packs (various ammonium salts) can. have you ever used a hot pack. Hot And Cold Packs Chemical Reaction.
From sciencenotes.org
Endothermic Reactions Definition and Examples Hot And Cold Packs Chemical Reaction hot pack cold pack (conservation of energy in chemical reactions, heat of solution, hess's law of heat summation) |. learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the. Hot And Cold Packs Chemical Reaction.
From www.slideserve.com
PPT Chapter 7 Hot & Cold Packs PowerPoint Presentation, free Hot And Cold Packs Chemical Reaction hot pack cold pack (conservation of energy in chemical reactions, heat of solution, hess's law of heat summation) |. learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the. Hot And Cold Packs Chemical Reaction.
From www.slideserve.com
PPT Hot Packs and Cold Packs PowerPoint Presentation, free download Hot And Cold Packs Chemical Reaction commercial heat packs (containing iron and water, or supersaturated sodium acetate) and cold packs (various ammonium salts) can. The sodium thiosulfate needs energy to. hot pack cold pack (conservation of energy in chemical reactions, heat of solution, hess's law of heat summation) |. have you ever used a hot pack to warm your hands or a cold. Hot And Cold Packs Chemical Reaction.
From www.youtube.com
Chemistry of Hot & Cold Packs YouTube Hot And Cold Packs Chemical Reaction learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. have you ever used a hot pack to warm your hands or a cold pack on an injury? How can something produce heat or cold without any microwaving or refrigeration involved? in this lesson plan, students will explore. Hot And Cold Packs Chemical Reaction.
From www.steroplast.co.uk
How do Hot and Cold Ice Packs Work? Hot And Cold Packs Chemical Reaction in this lesson plan, students will explore several endothermic and exothermic reactions, and use their observations to choose the chemical reaction that. learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. have you ever used a hot pack to warm your hands or a cold pack on. Hot And Cold Packs Chemical Reaction.